I am an MD/PhD candidate within the laboratory of David B. Beck at the NYU Grossman School of Medicine. My thesis work revolves around elucidating the mechanism of disease in patients with VEXAS Syndrome and characterizing the role of the master regulator of the Ubiquitin-Proteasome System (UPS), UBA1, in physiological and disease states.
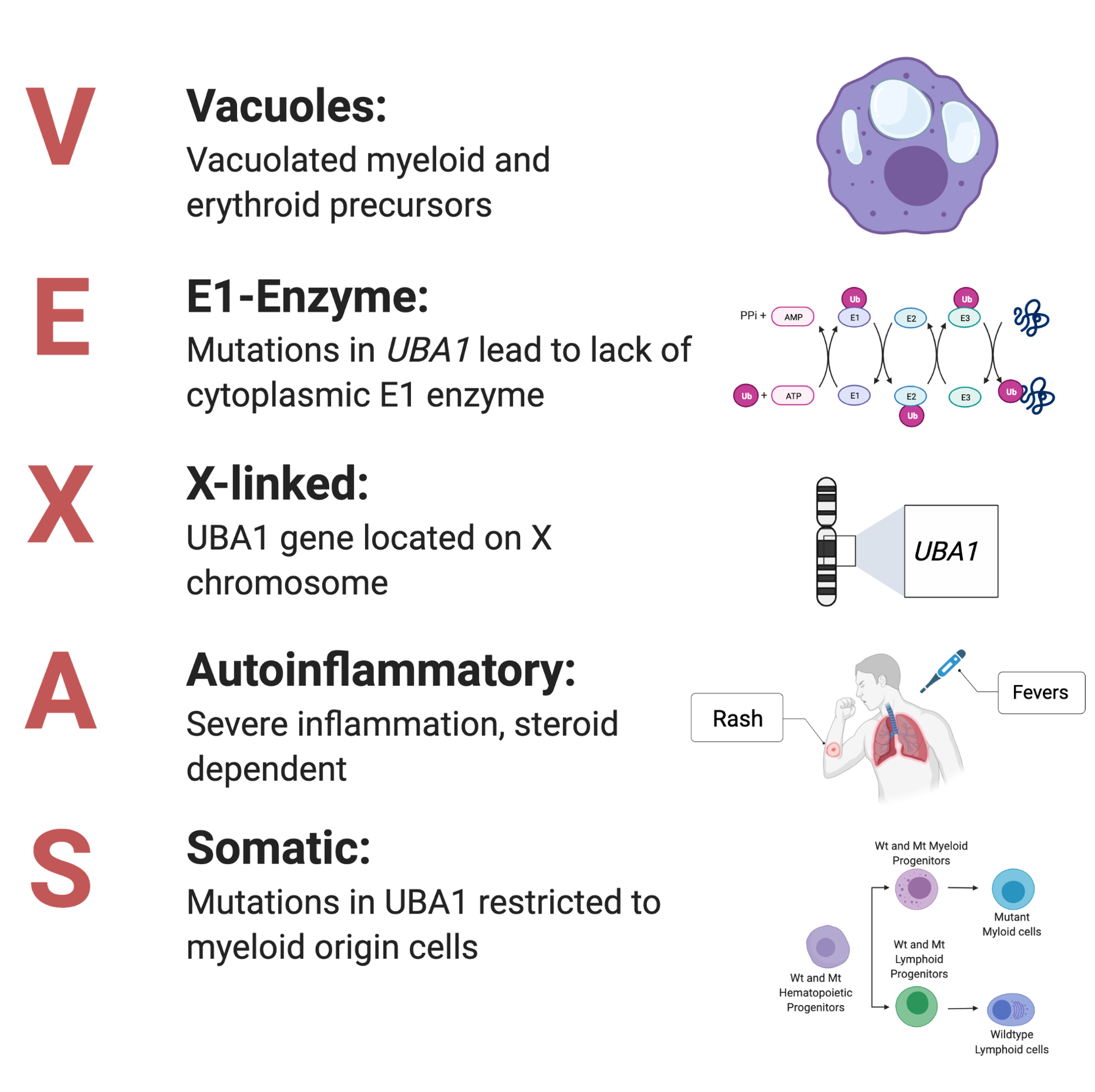
VEXAS was first described in 2020 and is itself is an acronym and stands for Vacuoles, E1, X-linked, Autoinflammatory, Somatic syndrome. As far as diseases go, VEXAS is new and thus there is much we do not yet understand. It presents with disparate inflammatory (relapsing polychondritis, polyarteritis nodosa, giant cell arteritis) and hematologic (myelodysplastic syndrome, plasma cell dyscrasia) conditions. In other words, these patients are very sick and the disease often proves fatal. The disease is present in 1:4000 men over the age of 50 and has a prevalence similar to hemophilia. In affected patients, somatic variants in the UBA1 gene are present in vacuole-forming hematopoietic stem cells (HPSCs). VEXAS presents primarily in elderly males as UBA1 is located on the X-chromosome. Presently, there is no cure other than remediation of the autoinflammatory symptoms through high dose corticosteroid treatment. However, many patients become refractory to these treatments making the identification of new therapies even more critical. My thesis is dedicated to identifying how this disease works so that we may better treat it. In addition, its study may shed light on other secrets…
UBA1 encodes the primary ubiquitin (Ub) activating enzyme (E1), accounting for over 97% of all downstream ubiquitylation. Cellular functions of ubiquitylation span a broad spectrum that includes proteolytic and nonproteolytic roles such as proteasomal degradation of proteins, embryogenesis, inflammatory signaling, autophagy, DNA repair, intracellular trafficking, receptor internalization, and enzymatic regulation. Deregulation of ubiquitylation enzymes has broad consequences. Through inappropriate activation or deactivation of pathways it may lead to aberrations in oncogenesis or cellular metabolism. Improper or insufficient Ub-dependent assembly of protein complexes is implicated in miscued inflammatory responses, faulty DNA repair, and autoimmune syndrome formation. In settings of a defunct ubiquitin proteasome system or subversion of Ub-dependent protein degradation, accumulation of misfolded proteins in the endoplasmic reticulum or cytosol leads to neurodegenerative diseases.
It is thus my hope that in elucidation of the mechanism of disease in VEXAS Syndrome, my work will not only identify therapeutic treatments for VEXAS patients but might also inform treatment for a wider spectrum of neoplasms and diseases.
Image Copyright: National Institutes of Health